Research
Technology-derived storage solutions for stabilizing insulin in extreme weather conditions I: the ViViCap-1 device

PMID: 28394190 DOI: 10.1080/17425247.2017.1316261
Abstract
Background: Injectable life-saving drugs should not be exposed to temperatures <4°C/39°F or >30°C/86°F. Frequently, weather conditions exceed these temperature thresholds in many countries. Insulin is to be kept at 4-8°C/~ 39-47°F until use and once opened, is supposed to be stable for up to 31 days at room temperature (exception: 42 days for insulin levemir). Extremely hot or cold external temperature can lead to insulin degradation in a very short time with loss of its glucose-lowering efficacy.
Methods: Combined chemical and engineering solutions for heat protection are employed in ViViCap-1 for disposable insulin pens. The device works based on vacuum insulation and heat consumption by phase-change material. Laboratory studies with exposure of ViViCap-1 to hot outside conditions were performed to evaluate the device performance.
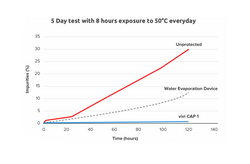
Results: ViViCap-1 keeps insulin at an internal temperature < 29°C/84.2°F for a minimum of 12 h without external power requirement, even when constantly exposed to an outside temperature of 37.8°C/100°F. Bringing the device into an ambient temperature < 26°C/78.8°F reverses the phase-change process and 'recharges' the device for further use.
Conclusions: ViViCap-1 performed within its specifications. The small and convenient device maintains the efficacy and safety of using insulin even when carried under hot weather conditions.
Keywords: Insulin injection; drug degradation; drug stability; extreme weather conditions.